Note that only electrons move - protons are still (hence the name)
This is charge by friction.
Materials can also be charged by induction.
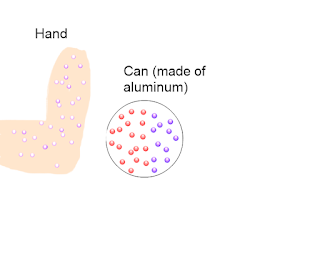
This is when a electrons are pushed away. Let's say that (for some reason) you are negatively charged. You place your hand in front of a can and it rolls towards you. How does this work? Because your hand is negatively charged, and like charges repel, it repels the electrons at the front of the can. This leaves the protons, which are positively charged. Therefore, the can is now charged positively by induction. Because opposite charges attract, the can rolls towards your hand.
To demonstrate this visually (sorry about the bad drawing skills, that was me making the diagrams)
Key - purple dots are electrons and red dots are protons (note that on both diagrams, the hand is supposed to have electrons on it but I didn't quite get the same shade of purple the second time through)
 |
| Figure 1 - Step 1 |
 |
| Figure 2 - Step 2 |
No comments:
Post a Comment